NMN Rejuvenates Bone Tissue Stem Cells
The reagent nicotinamide mononucleotide (NMN) docks onto the nucleosome assembly protein 1 like-2 (NAP1L2) to reinvigorate the “stemness” of bone mesenchymal stem cells.
Highlights
- The study suggests that NMN binds to the SIRT1 protein to activate genes for bone regeneration (osteogenesis).
- By stimulating osteogenesis, NMN may prevent the progression of osteoporosis.
The breakdown and generation of new bone cells (osteogenesis) declines as people age. The disarray of bone regeneration stems from depleted stem cells in their original, youthful state (stemness). The bone stem cells’ stemness declines due to the accumulation of mutations in the stem cells as they progress through the cell cycle to maturity as osteoblasts.
In other words, as stem cells become osteoblasts, bones harden, may fracture easily, and develop pores (osteoporosis), leading to frailty. Clinicians can measure frailty using the Frailty Index, a number that is a proxy to the state of an elderly individual’s overall fitness and health. For these reasons, research has been geared toward finding ways to maintain stemness of bone mesenchymal stem cells (BMSCs), the stem cells that produce osteoblasts and, concomitantly, bone tissue.
NMN Genetically Refurbishes Elderly Human Bone Stem Cells
Previous research has shown that the immediate precursor to nicotinamide adenine dinucleotide (NAD+), nicotinamide mononucleotide (NMN), restores bone tissue health in rodents like mice and rats. With that being said, Liu and colleagues from Tianjin Medical University utilized BMSCs from young and old patients to test whether administering NMN restores the epigenetic code, the youthful pattern of gene expression, in these cells. In doing so, the Tianjin-based research team found that the BMSCs undergoing chemotherapeutic treatment and oxidative stress showed enhanced stemness after being treated with NMN. Interestingly enough, the China-based team reported further data showing that NMN binds to DNA to potentially rejuvenate gene expression profiles.
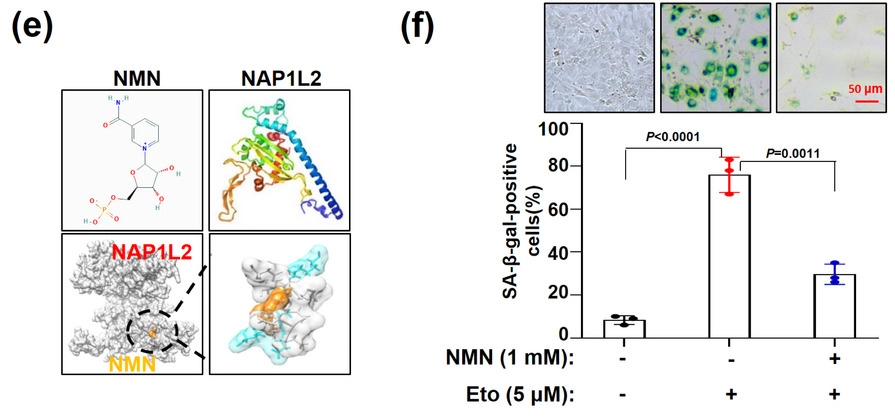
Increased expression of the protein nucleosome assembly protein 1-like 2 (NAP1L2) correlates with aging. Liu and colleagues hypothesized that with increased NAP1L2 levels, this protein, a nucleosome chaperone, removes molecular tags called acetyl groups from proteins that DNA wraps around (nucleosomes). NAP1L2 also recruits the deacetylase protein Sirtuin1 (SIRT1) that removes acetyl groups from promoter regions of genes that drive osteogenesis.
NAP1L2 Protein Levels Increase with Age, Correlating with Frailty
To test this idea, the Tianjin-based team sought to link increased NAP1L2 protein expression with senescence in BMSCs from elderly adults. To measure senescence, the researchers measured senescence with the molecular marker β-galactosidase. They also found dysfunction of the chromosome ends (telomeres). What’s more, their analyses of elderly BMSC protein levels revealed senescence associated secretory phenotype (SASP) markers after stressing the BMSCs with hydrogen peroxide (H2O2), in tandem with increased NAP1L2 levels. These results indicate that BMSC senescence and loss of stemness occur in tandem with elevated NAP1L2 levels.
NMN Binds to NAP1L2 to Stimulate the Cell-Rejuvenating SIRT1 Protein
To then find out how NMN affects NAP1L2 levels in BMSCs from elderly adults, Liu and colleagues treated BMSCs in laboratory dishes with a 1 mM concentration of NMN. The research team found that, intriguingly, NMN docks with the NAP1L2 protein and also alleviates the accumulation of the senescence-associated β-galactosidase. Not only that, but NMN treatment alleviated the accumulation of telomere DNA damage in the senescent BMSCs. Moreover, treating the cells with NMN suppressed the expression of SASP factor genes. These results indicate that NMN rejuvenates BMSCs, at least in laboratory dishes. If these findings hold weight in live humans, it could mean that using NMN restores the “stemness” of BMSCs to possibly restore osteogenesis and alleviate osteoporosis as humans age.
Further evidence confirming this notion comes from a human clinical trial, showing that NAP1L2 level elevations correlate with osteoporosis in the elderly. For example, a negative correlation was found between NAP1L2 expression and bone density, meaning that as we age and NAP1L2 levels increase, bone becomes more porous. Although further testing is needed, the possibility remains that supplementing with NMN could increase bone density in humans to make bones stronger. The promise of the NMN reagent means that humans could benefit from supplementing with the compound to potentially reverse Frailty Index scores as we grow older. In doing so, we may live healthier lives and hopefully alleviate sorrow and despair in elderly individuals. Perhaps by improving overall feelings of wellbeing, older individuals who take NMN could have better outlooks on life, providing a potential source of optimism.

